The big equations:
CaCO3 ⇌ Ca2+ + CO32-
CaCO3 (s) + H2CO3 (aq) ⇌ Ca2+ (aq) + 2HCO3- (aq)
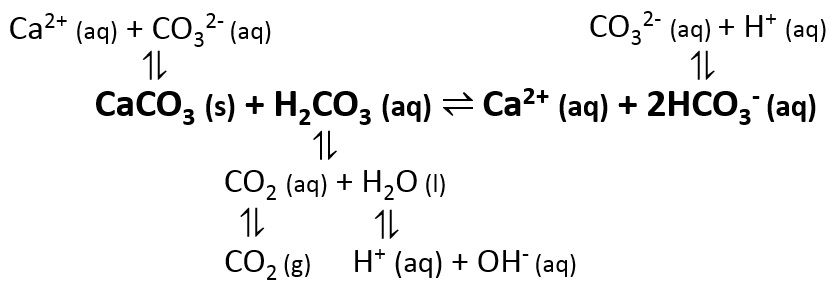 For refreshers on chemistry concepts like Qc and Kc, see these websites:
For refreshers on chemistry concepts like Qc and Kc, see these websites:
- The Equilibrium Constant
- Difference Between K And Q
- SkepticalScience on ocean acidification (make sure you go through all of the mini-pages)
Ω: what we have
![\Omega = \frac{\text{have}}{\text{need for saturation}} = \frac{\text{[Ca}^{2+}\text{ present}][\text{CO}_{3}^{2-} \text{ present}]}{\text{[Ca}^{2+}\text{ needed}][\text{CO}_{3}^{2-} \text{ needed}]}](https://www.luckysci.com/wp-content/plugins/latex/cache/tex_eeb9611a3c34c7c9c9ef5dd9723a41f4.gif)
CaCO3 ⇌ Ca2+ + CO32-
Note: Ca2+ is supplied by weathering.
Ω tells us how much we have relative to what we need for a saturated solution.
Ω tells us how CaCO3 will behave.
Ω > 1 Precipitation
Ω = 1 Equilibrium
Ω < 1 Dissolution
Modern Sea Surface Ω ≈ 2-5
Sea surface is supersaturated with respect to CaCO3,
but calcium carbonates are not constantly precipitating.
In the ocean, Ω decreases with depth.
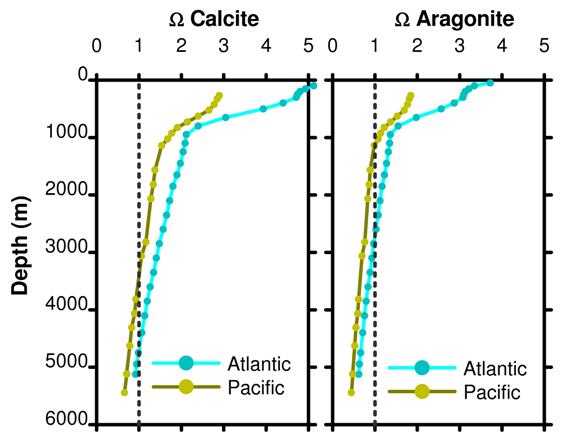 Image credit: http://www.skepticalscience.com/print.php?n=933
Image credit: http://www.skepticalscience.com/print.php?n=933
Kc: what we want
At 25° C:
Calcite Kc = 10-8.35
Aragonite Kc = 10-8.22
![\Omega = \frac{\text{[Ca}^{2+}][\text{CO}_{3}^{2-}] }{[\text{CaCO}_3]} = \frac{1}{10^{8.35}} = 10^{-8.35}](https://www.luckysci.com/wp-content/plugins/latex/cache/tex_53276f8d9b416b05dedf427037d85cd2.gif)
When the concentration ratio of products (numerator) to reactants (denominator) is equal to Kc, the reaction is at equilibrium; the concentrations of the products and reactants remain the same. The reaction is not favored in either the forward or backward direction.
What if Ω > K?
![\Omega = \frac{[\text{products}]}{[\text{reactants}]} = \frac{\text{[Ca}^{2+}][\text{CO}_{3}^{2-}] }{[\text{CaCO}_3]} = \frac{10}{1}](https://www.luckysci.com/wp-content/plugins/latex/cache/tex_b278aba6275e14a5ba6df2014b9abbc4.gif)
In this example, the balance is "top heavy" compared to the equilibrium value (Kc), where there are 1 products for every 108.35 reactants.
This means that in our example, where we have 10 products for every 1 reactant, there are too many products (Ca2+ and CO32-) compared to Kc. To achieve equilibrium, the amount of CaCO3 must increase, and it will increase until it reaches the number that will give us a ratio of 1:108.35. So, in our example, the reaction will run backward -- toward CaCO3. Therefore, CaCO3 will precipitate when Ω > K.
CaCO3 ⇌ Ca2+ + CO32-
Extra CaCO3 stops precipitating when equilibrium is reached:
![\Omega = \text{K} = \frac{[\text{products}]}{[\text{reactants}]} = \frac{\text{[Ca}^{2+}][\text{CO}_{3}^{2-}] }{[\text{CaCO}_3]} = \frac{10}{10^{9.35}} = \frac{1}{10^{8.35}}](https://www.luckysci.com/wp-content/plugins/latex/cache/tex_7b8967c751272540ae3d2620c4f8d09e.gif)
If we switched the values so that Ω < K -- in other words, we had too much CaCO3 -- then the reaction runs forward: CaCO3 will dissolve.
How do we get carbonate?
Total Dissolved Inorganic Carbon =
[CO2] + [HCO3-] + [CO32-] + [H2CO3]
carbon dioxide + bicarbonate + carbonate + carbonic acid
CO2 (g) ⇌ CO2 (aq)
CO2 (aq) + H2O (l) ⇌ H2CO3 (aq)
H2CO3 (aq) ⇌ HCO3- (aq) + H+ (aq)
HCO3- ⇌ CO32- (aq) + H+ (aq)
The last two reactions release H+, so,
CO2 in water lowers pH
CO2 out of water raises pH
The Ideal Gas Law states that:
PV=nRT
pressure × volume = amount (moles) × ideal gas constant × temperature
When pressure is higher, more CO2 dissolves into the water .
When temperature is lower, more CO2 dissolves into the water.
(At lower pressures and higher temperatures, CO2 can escape as a gas.)
Note that carbon speciates in the water depending on the water pH:
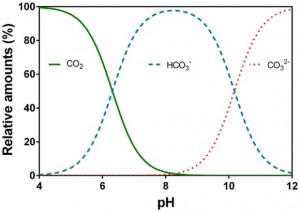 Image credit: Pedersen et al. (2013)
Image credit: Pedersen et al. (2013)
So what controls CaCO3 solubility?
Lower solubility means more CaCO3 precipitates!
↑ Concentration CaCO3 = Solubility ↓
↑ Temperature = Solubility ↓
↓ Pressure = Solubility ↓
↓ Other Ions in Solution* = Solubility ↓
*The increasing availability of ions that can tie up either Ca2+ or CO32- will lowers the effective concentration of Ca2+ or CO32- and increases solubility, making it more difficult for Ca2+ and CO32- to combine and precipitate. Examples: CaCl2, CaSO4, MgCO3, Na2CO3
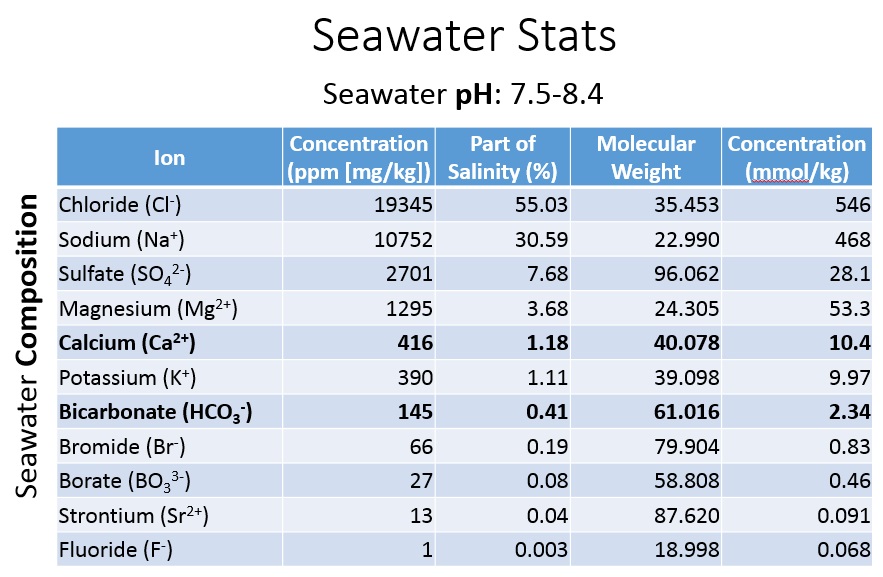
Modern seawater has lots of dissolved ions!
Putting it all together
The big equation:
CaCO3 (s) + H2CO3 (aq) ⇌ Ca2+ (aq) + 2HCO3- (aq)
To dissolve calcium carbonate
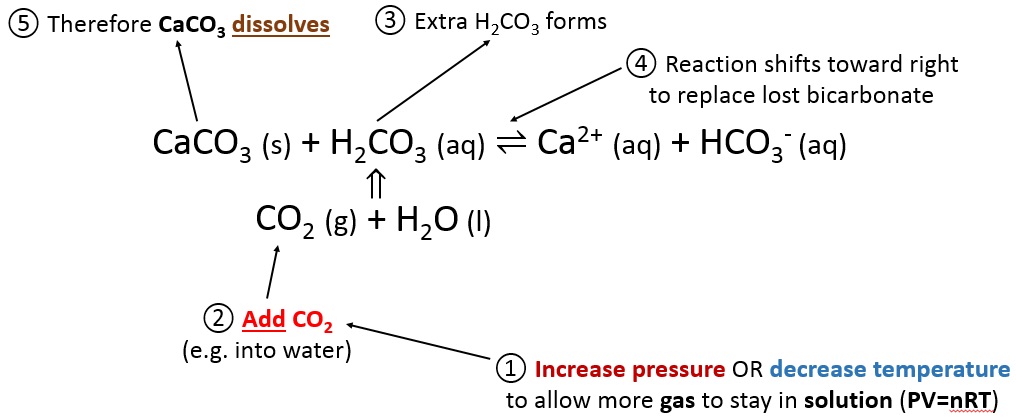 CaCO3 (s) + H2CO3 (aq) ⇌ Ca2+ (aq) + 2HCO3- (aq)
CaCO3 (s) + H2CO3 (aq) ⇌ Ca2+ (aq) + 2HCO3- (aq)
To dissolve calcium carbonate:
- Increase pressure or decrease temperature
- Per the ideal gas law, this allows more CO2 to enter the water
- The extra CO2 creates more H2CO3
- There is too much H2CO3 for equilibrium
- The reaction must go to the right to get rid of the extra H2CO3
- CaCO3 dissolves
To precipitate calcium carbonate
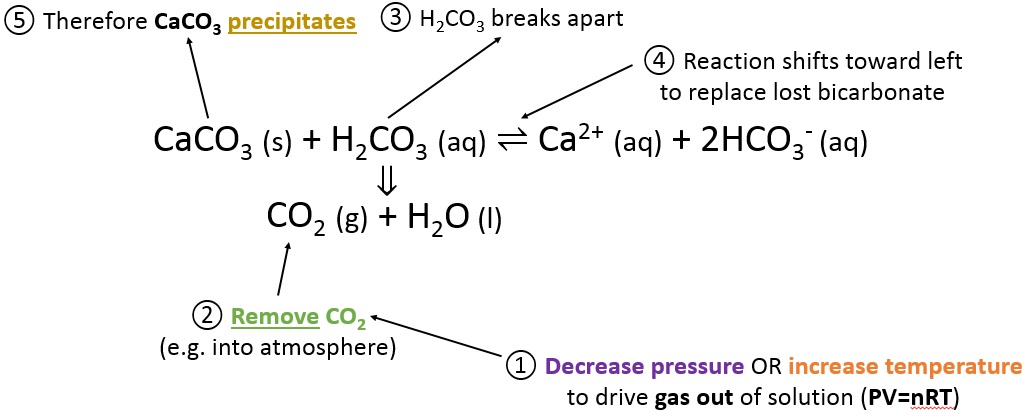 CaCO3 (s) + H2CO3 (aq) ⇌ Ca2+ (aq) + 2HCO3- (aq)
CaCO3 (s) + H2CO3 (aq) ⇌ Ca2+ (aq) + 2HCO3- (aq)
To precipitate calcium carbonate:
- Decrease pressure or increase temperature
- Per the ideal gas law, this drives CO2 out of the water
- H2CO3 breaks down to release the CO2
- There is too little H2CO3 for equilibrium
- The reaction must go to the left to replace the lost H2CO3
- CaCO3 precipitates
How can we decrease pressure?
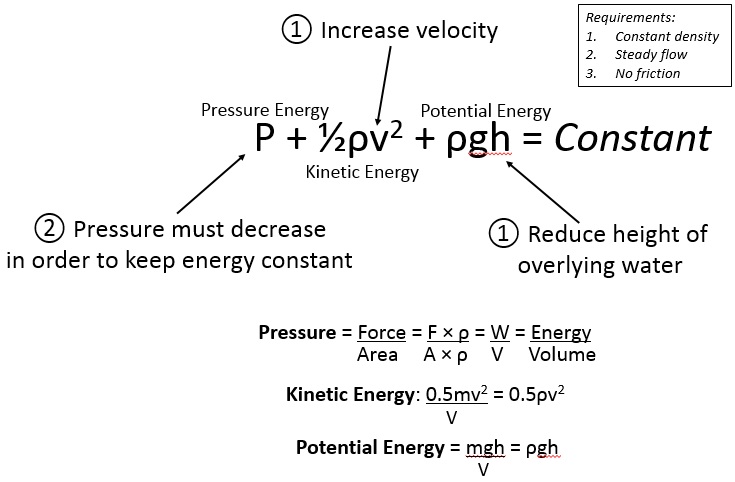 Bernoulli's principle states that:
Bernoulli's principle states that:
Pressure + Kinetic Energy + Potential Energy = Constant
P + ½ρ2 + ρgh = Constant
So if you increase kinetic energy (waves), then pressure must go down.
Coral connection
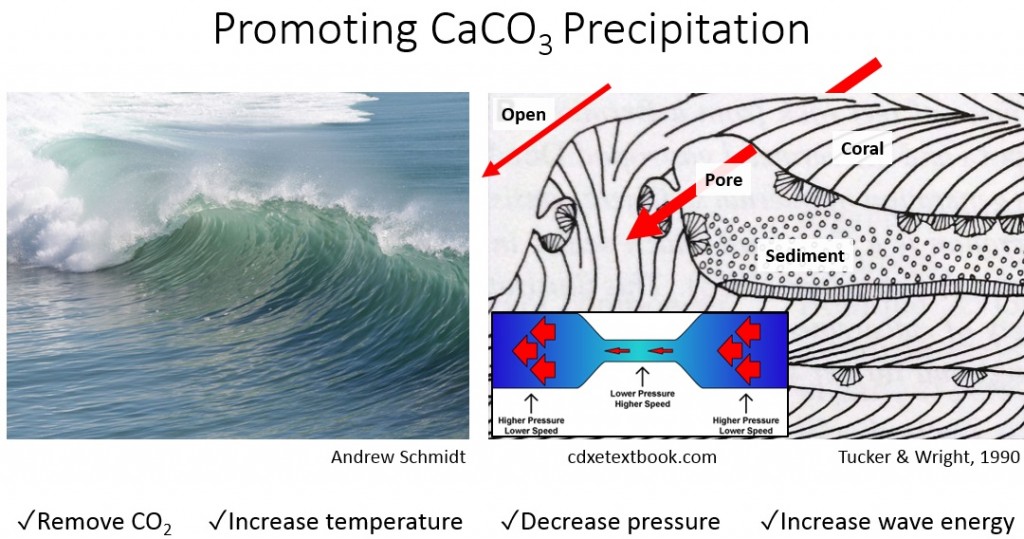 As producers of calcium carbonate (this is what comprises their skeletons), corals like conditions that favor CaCO3 precipitation. Corals like:
As producers of calcium carbonate (this is what comprises their skeletons), corals like conditions that favor CaCO3 precipitation. Corals like:
- Wave action drives off CO2 by lowering pressure.
- High temperatures drives off CO2 by lowering pressure.
- Less acidic means less carbon is in CO2 form.
- They also require sunlight for their nutrient-sharing symbionts to photosynthesize, so they like shallow and clear water.
What about beackrock cementation?
Beachrock is a limestone that forms underwater along the shoreline. It usually dips seaward. It mostly occurs along tropical-temperate, windward coasts. Its formation is still being debated!
- Direct precipitation from seawaters and sea sprays
- CaCO3 precipitates due to intertidal alternate wetting and drying, which allows seawater to evaporate
- High energy in certain coastal areas favors precipitation
- Nucleation around pre-existing beach sediments
- Issues: beachrock in arid areas
- Precipitation due to marine and fresh water mixing in coastal water tables
- Solubility of CaCO3 decreases with salinity, as pure water has less ions to tie up Ca2+and CO32-
Issues: beachrock on islands too small to have freshwater tables
- Solubility of CaCO3 decreases with salinity, as pure water has less ions to tie up Ca2+and CO32-
- Precipitation due to CO2 de-gassing of coastal groundwater
- As groundwater reaches the surface (lower pressure) and experiences tidal pumping, CO2 de-gasses
- Biological activity
- For example, carbon fixation by cyanobacteria removes dissolved inorganic carbon from the water, raising the local pH
Summarized, in part, from my handwritten notes taken during Dr. Terri Woods' GEOL 540 course at ECU in Fall 2014.