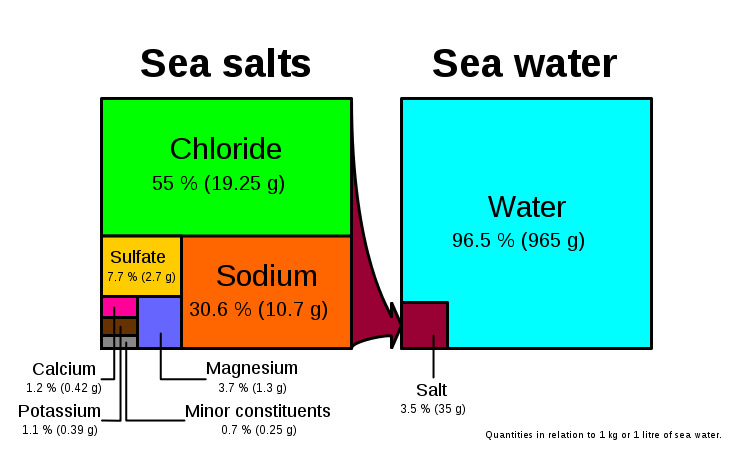
Salts form 3.5% (more commonly stated as 35 ppt) of seawater by mass. If you evaporate seawater, the following minerals precipitate out in this order (the reverse of their solubility):
1) Calcite (CaCO3)
2) Gypsum (CaSO4 * 2H2O)
3) Halite (NaCl)
4) Sylvite (KCl)
You can remember the order because it's alphabetical!
 Large deposits of evaporites can form during the birth of ocean basins. When the Gulf of Mexico began opening in the Early Jurassic (180 mya), seawater aperiodically flooded the foundling basin via gaps between the splitting Yucatan and Florida-Bahama Blocks; the trapped seawater would evaporate, leaving behind evaporites, and then the cycle would eventually repeat. Ultimately, a 4 km layer of evaporites covered the Gulf's floor before it permanently flooded in the Early Cretaceous (180 mya).
Large deposits of evaporites can form during the birth of ocean basins. When the Gulf of Mexico began opening in the Early Jurassic (180 mya), seawater aperiodically flooded the foundling basin via gaps between the splitting Yucatan and Florida-Bahama Blocks; the trapped seawater would evaporate, leaving behind evaporites, and then the cycle would eventually repeat. Ultimately, a 4 km layer of evaporites covered the Gulf's floor before it permanently flooded in the Early Cretaceous (180 mya).
Similar deposits formed via similar processes all over the world. An excellent modern example of evaporites lies inside West Caicos, a small island in Turks & Caicos. The salina is flanked to the west by an oolitic grainstone Pleistocene ridge and to the east by Holocene sand dunes.