How were the elements made? What explains the relative abundance of each element in our solar system? Below you will find a very simplified overview of nucleosynthesis, the process by which elements are formed in the burning hearts of stars. For additional information, start here.
Solar system elemental abundance
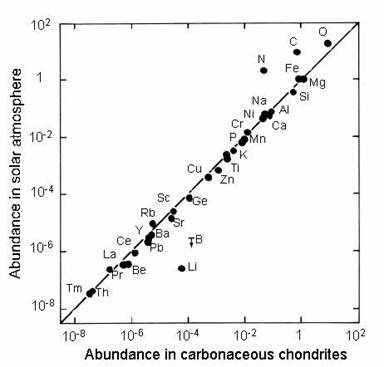
The abundances of the elements in our solar system were estimated using meteorite compositions and spectral analyses (emissions) of stellar objects. These two lines of evidence, which closely corroborate each other.
Chondritic meteorites contain chondrules, rounded grains that formed as molten droplets during the solar system's youngest days. Since they formed very early and have not undergone alteration or differentiation, chondrules tell us about the formation and composition of the solar system.
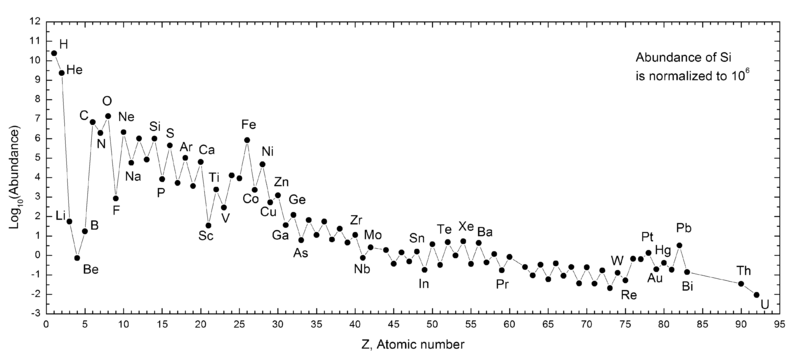
Image credit: 28bytes, Wikimedia
Observations
- H and He are by far the most common.
- Li, Be, B are depleted.
- C, N, and O are elevated.
- There is a serrated pattern.
- Fe and Pb are elevated.
Explanation
- Before the Big Bang (13.8 bya), 90% of atoms were H, with He making up the rest. Today, thanks to their head start, H and He are still the most common atoms.
- Li, Be, and B made through normal nucleosynthesis decay extremely rapidly. The stable forms are made by spallation.
- The C-N-O cycle explains why those three elements are elevated.
- The serrated pattern results because even atoms are more common than odd!
- Within the nucleus, protons are arranged in layers. An even number of protons allows proton pairs to spin in opposite directions, which makes them more stable. The opposite spins allow protons to crowd closer together, resulting in higher binding energy.
- This also applies to isotopes, so even isotopes are more common than odd; for example, tin-120 is more abundance than tin-119.
- There are 157 stable nuclides with an even mass and even number of protons and neutrons; 53 with an odd mass and and even protons and neutrons; 50 with an odd mass, odd protons, and even neutrons; 4 with an even mass and odd protons and neutrons.
- Iron has the highest binding energy per nucleon (a proton or neutron), resulting in its disproportionate abundance. Furthermore, some atoms decay to iron.
- The "magic numbers" of nucleons that favor higher abundance are 8, 10, 20, 28, 50, 82, and 126.
Creating the elements
All elements form in the hearts of big stars, with hotter stars capable of forming heavier elements. A star the sun's size specializes in helium production and can make elements only up to nitrogen!
Hydrogen Fusion
Protiums smash together to create deuterium. Deuterium collides with protium to create helium-3. Helium-3 combine to create helium-4 (an alpha particle).
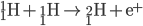
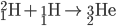
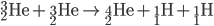
Once first generations stars (the first stars to form in the universe) collapsed, second generation stars used the dead stars' helium to begin creating heavier elements via the C-N-O cycle.
Triple Alpha Process
Three alpha particles lead to carbon.
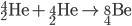
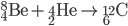
Fusion
Inside the centers of huge stars, alpha particles combine with heavier nuclides to create even heavier nuclides, up to iron-56. After 56Fe, fusion is not energetic enough to form heavier nuclides. Temperatures on the order of 109 K are needed to make silicon-28.
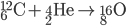
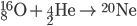
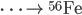
See the additional reactions here.
Neutron Capture
A supernova ultra-energizes neutrons, leading to elements as heavy as uranium-238. This is also called the r process. Supernovas, which occur when stars collapse, have cores of100 billion K!
Spallation
Stable forms of lithium, beryllium, and boron are not formed by normal nucleosynthesis, but rather spallation, which involves cosmic rays hitting gas.
Summarized from my handwritten notes taken during Dr. Terri Woods' GEOL 540 course at ECU in Fall 2014.